Yupo
What is Yupo?
Paper, Paint and Plastic . . .
Watercolor painting is a technique that predates recorded history, but its common use as an art medium can be dated to the Renaissance. Even though artists working in other mediums commonly employed watercolors only for sketches, copies or preliminary studies, contemporary watercolor painting has shed its “unloved stepchild” status within the art world to attract devoted professionals who eschew other mediums while creating monumental works of art.
For many, the attraction of painting with watercolors is in the use of simple materials—paint, water, and paper—that are generally non-toxic and easily obtained. The seemingly unpredictable nature of how paint flows on wet paper can be both terrifying and exhilarating, which explains why many artists have a love-hate relationship with this medium. Artists who love watercolor the most are those who can “go with the flow”, so to speak. The use of new painting surfaces such as Yupo adds to the excitement of creating work having a look never before seen.
Paper
The beginning of modern-day paper making can be traced to ancient China. Early paper was thin and transparent, and the production of thicker stock like the paper used today was the contribution of the Arab peoples in Samarkand, an important city on the “Silk Road” that linked Europe with Asia. The first paper mill was founded there. Below are listed some milestones in the history of paper making.
- 105 A.D: Start of paper making from pulp (Mulberry bark)
- 751: Invention spreads to the Middle East
- 1151: First record of a paper mill in Europe (Spain)
- 1276: Paper manufacture in Italy
- 1588: Commercial paper making in England
- 1844: Process established to make paper from wood pulp
Before the use of wood pulp, paper was made from recycled rags (cotton or linen), hence the term “100% cotton rag” paper. Cotton is 91% cellulose, the chemical structure of which is shown below (black dots represent carbon atoms). The structures of glucose and fructose are also shown below. All of these compounds are carbohydrates [literally, “hydrates of carbon”, so-called because the ratio of carbon atoms to water molecules is 1:1; that is, these substances have the general chemical formula of Cn(H2O)n. For example, both glucose and fructose have the formula, C6(H2O)6]. Many simple carbohydrates are called “sugars” or “saccharides.”

Cellulose is a polymer (polymer means “many units”) of glucose, which is the most abundant organic molecule on earth. Glucose is a sugar (saccharide); cellulose is a polysaccharide.
Wood consists largely of cellulose and lignin, a polymer made of molecules that are quite different in structure from those of sugars. Lignin is the material that makes trees rigid; otherwise, they would be like cotton!
If wood is mechanically processed to form pulp, then the lignin is still present. This type of pulp manufacturing is used to make newsprint. Because of its acidity, the residual lignin is responsible for newsprint’s rapid yellowing.
To make better grades of paper, the lignin has to be removed chemically. The resulting pulp, which is mostly cellulose, still requires bleaching before being made into white paper. To make the paper pH neutral, acidic contaminants have to be neutralized as well. Adding an excess of a neutralizing substance—often calcium carbonate (“chalk”)—creates an “alkaline reserve” so that the paper can respond to and counteract any acidic impurities from the environment in which it is used.
Watercolor paint
Watercolor paints consist of a pigment and gum arabic, which is a binder that holds the pigment in suspension and fixes the pigment to the painting surface. Water and other substances such as honey are added to modify the viscosity of the paint.
Gum arabic is a complex polymer comprising several different carbohydrates, mainly galactose and arabinose. A partial structure of gum arabic, shown below, is similar to that of cellulose, which is why paper and watercolor paints are so well suited to each other.
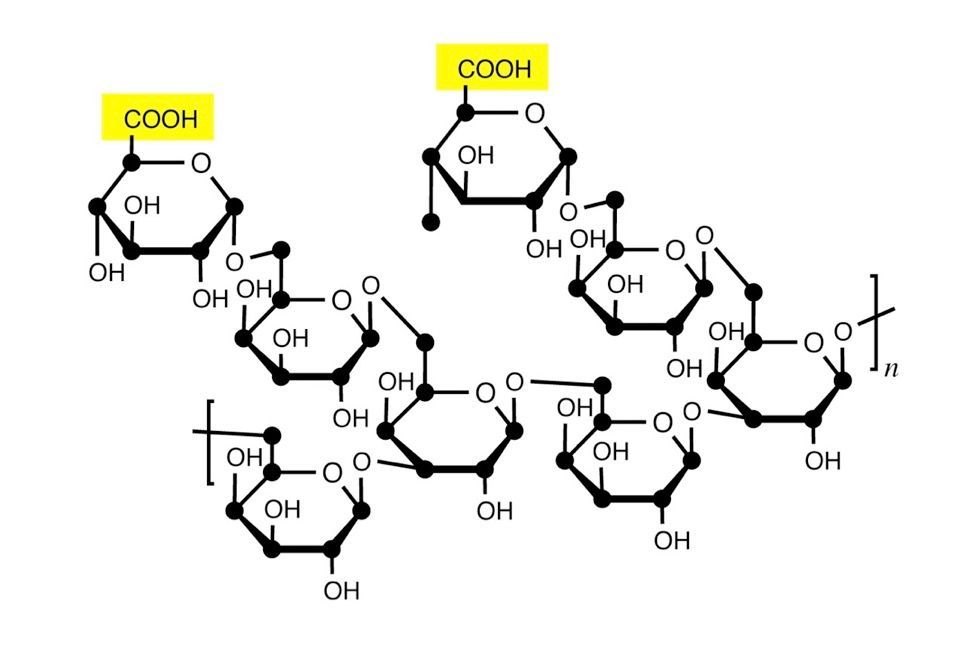
This figure oversimplifies the actual structure of gum arabic, which also has protein components. The acidic groups in gum arabic (shown in color) are associated with gluconic acid (an oxidized form of glucose), and these acid groups can form salts with metal ions. In watercolor painting, these acid groups react with the alkaline reserve of the paper, so you don’t have to worry about watercolor paints damaging your pH-neutral paper.
Honey is a mixture mainly of fructose, glucose, and water, which makes it an ideal additive to watercolors because it is compatible both with gum arabic and cellulose (or paper). I hope that you can begin to appreciate that many of the chemical substances associated with watercolor painting are interrelated and highly compatible.
Plastic
In 1968, concerns about the future availability of wood pulp led the Science and Technology Agency of Japan to recommend developing “synthetic paper” derived from petrochemical feedstock rather than from wood pulp. Yupo was one type of synthetic paper developed as a result of this challenge, and it is made by adding appropriate fillers to a synthetic resin, followed by fusion, kneading and extrusion. The fused resin is cooled temporarily, heated to re-soften it, and then stretched in both longitudinal and cross directions to form a film (the biaxial oriented film method). During stretching, tiny holes (micro-voids) are encouraged to form.
Yupo is made of polypropylene, which is a hydrocarbon (a molecule that contains only hydrogen and carbon). The black dots in the following structure represent carbon atoms (with either 1, 2 or 3 hydrogen atoms attached). You can see that its structure is quite different from cellulose and gum arabic in that there are no oxygen atoms and no rings.

Polypropylene is a polymer of propylene, which is a gaseous molecule (C3H6) at room temperature. Polypropylene is a solid with long chains of carbon atoms that can be spun into thread to make apparel (many hiking garments are made of polypropylene because it has the property of “wicking away” moisture) or formed into shapes, including sheets such as Yupo. Among other additives, Yupo contains calcium carbonate, which is also the “alkaline reserve” found in many papers. How polypropylene and calcium carbonate are combined to make Yupo is proprietary, but that combination, in addition to the micro-voids formed during the biaxial oriented stretching process, has much to do with why Yupo works as a painting and printing surface.
To see how different Yupo is from polypropylene itself, try painting with watercolors on any piece of plastic with the “5” recycling symbol stamped into it. The feel and results are completely different from what you experience with Yupo.
Some artists have raised concerns about the longevity of paintings done on Yupo. We will not know for certain until a suitable amount of time has passed, but we can make an assessment based on what we know of the chemistry underlying the ingredients of Yupo. Hydrocarbons as a chemical class are notoriously unreactive. Consider petroleum or natural gas, both of which have been around for eons and are broken down in the environment only by bacteria, yeast, and fungi. Polypropylene will react with oxygen and also with UV light, but those degradation processes are so slow that it would take centuries to detect any decomposition. Likewise, calcium carbonate is inert. Think about the longevity of seashells and chalk. Calcium carbonate does react with acids, but then so does cellulose. As long as your paints and framing materials are pH neutral, you should have no problem with degradation of the painting surface itself.
The more questionable issue about painting on Yupo with watercolors is how long the pigment will stick to the surface. When painted on paper, watercolor paints adhere naturally because of the structural similarities of cellulose and gum arabic. This same compatibility does not exist between watercolor paints and polypropylene, which is why you can readily wipe paint from Yupo and return to a white surface.
The micro-voids generated during the stretching process probably create defects in the otherwise impenetrable surface so that paint can adhere, but this adhesion must be weak because the paint can be washed off with water.
The calcium carbonate in Yupo probably also alters the surface to provide better adhesion. Because gum arabic has acidic groups, calcium carbonate can react with those groups to form the calcium salt of gum arabic plus carbon dioxide and water. If this reaction takes place, there is less likelihood that the paint will be lost because of the attractive forces between the positive calcium ion and the negative charges of the acid groups (opposite charges attract).
Spraying the finished painting with a fixative will also help keep the paint in place because the paint is protected from interaction with water; but as yet, there are no long-term studies on that matter. Only time will tell.
Anyone concerned about painting on Yupo should ponder this question: Did Da Vinci, Rubens, Rembrandt, or myriad other artists know for sure how many centuries their works would be able to endure? Despite all of their secret material formulas and highly trained surface preparations, they too probably expressed concerns and contemplations similar to today’s Yupo enthusiasts.